Our Pipeline
We have several diagnostic and therapeutic programs focused on a range of neurodegenerative diseases.
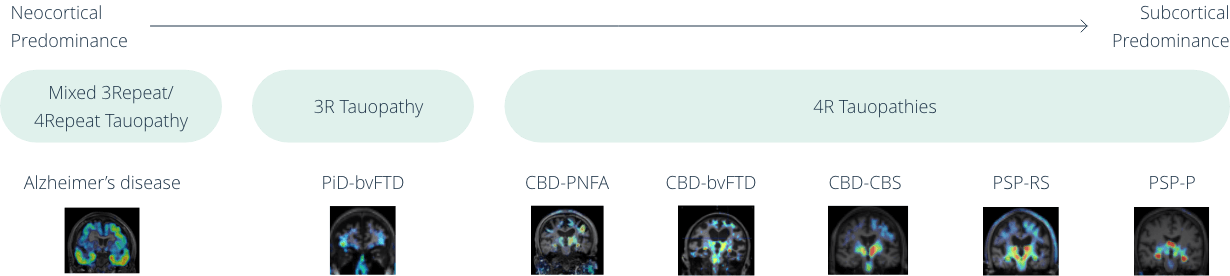
[18F]-APN-1607 (florzolotau 18F) recognizes pathological tau aggregates in all tauopathies, such as Alzheimer’s disease (AD), PSP, CBD, and PiD, providing a unified positron emission tomography (PET) imaging solution for those diseases. With a single PET scan, [18F]-APN-1607 provides a high-resolution, quantifiable 3-D map revealing the brain distribution of toxic tau protein in all kinds of tauopathies, conveying key information for clinicians to conduct differential diagnoses. This distinctive feature makes [18F]-APN-1607 the only tau tracer known to date that is able to distinguish early vs late onset of AD, as well as detect rare degenerative diseases, including PSP, FTD, PiD (Pick’s disease). [18F]-APN-1607 has been clinically validated in more than 2000 patients and demonstrated correlation with disease scores in AD and PSP patients. Accurate diagnosis provided by [18F]-APN-1607 will enable better personalized treatments and smarter clinical trial designs for novel therapies.
APRINOIA initiated a phase 2, multicenter, multinational study of [18F]-APN-1607 in the US, Taiwan, and Japan in November 2019, while phase 3 clinical trials were approved by China’s National Medical Products Administration in October 2020.
Candidates from this program are designed to reveal the brain accumulation of pathological α-synuclein proteins, a hallmark for alpha-synucleinopathies. This project is being funded by the Michael J. Fox Foundation, Lundbeck, and AbbVie.
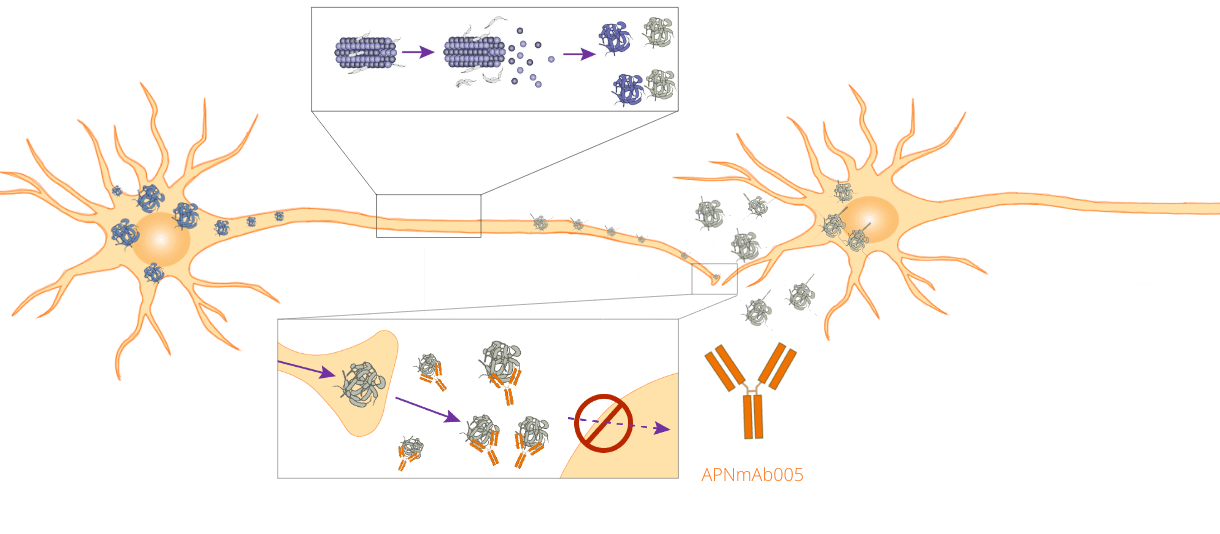
APNmAb005 is our antibody program’s lead candidate. A unique feature of APNmAb005 is that it can accurately identify and capture pathological tau species present in the AD brain tissues, but not healthy brain tissues. Since APNmAb005 can capture pathological tau species being transmitted through synaptic compartments, it may prevent extracellular tau aggregates from spreading into adjacent neurons, thus alleviating the progression of tauopathies.
APNmAb005 recognizes multiple 3-D epitopes that are only present in aggregated tau oligomers, not in normal tau protein. This unique feature confers selectivity to toxic tau species and differentiates APNmAb005 from other antibodies that also recognize normal tau proteins.
APRINOIA received FDA’s clearance in March 2022 to proceed with Phase 1 clinic trial in the US. The trial is currently under active recruitment to evaluate APNmAb005’s safety in healthy subjects.
Candidates of our tau modulator program bind to pathological tau aggregates and modulate their assembly, converting the tau aggregates into harmless conformations to revive the functions of the nervous system.
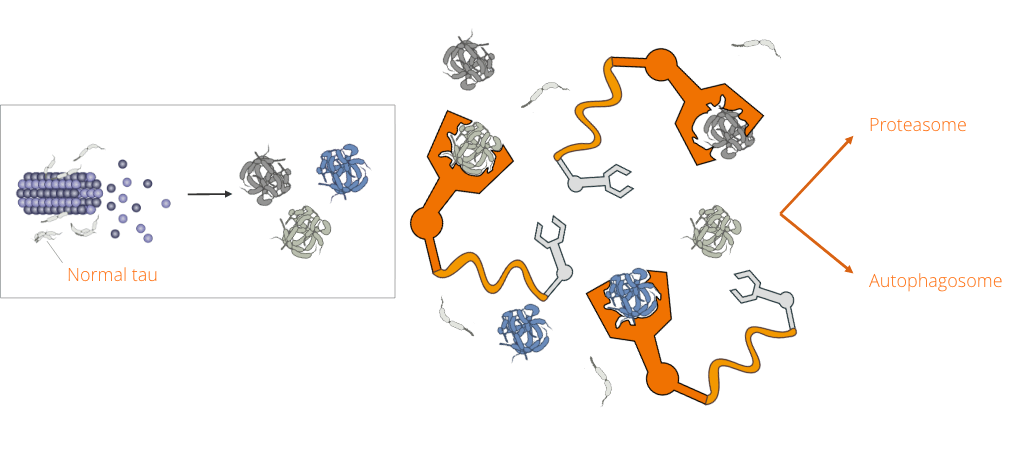
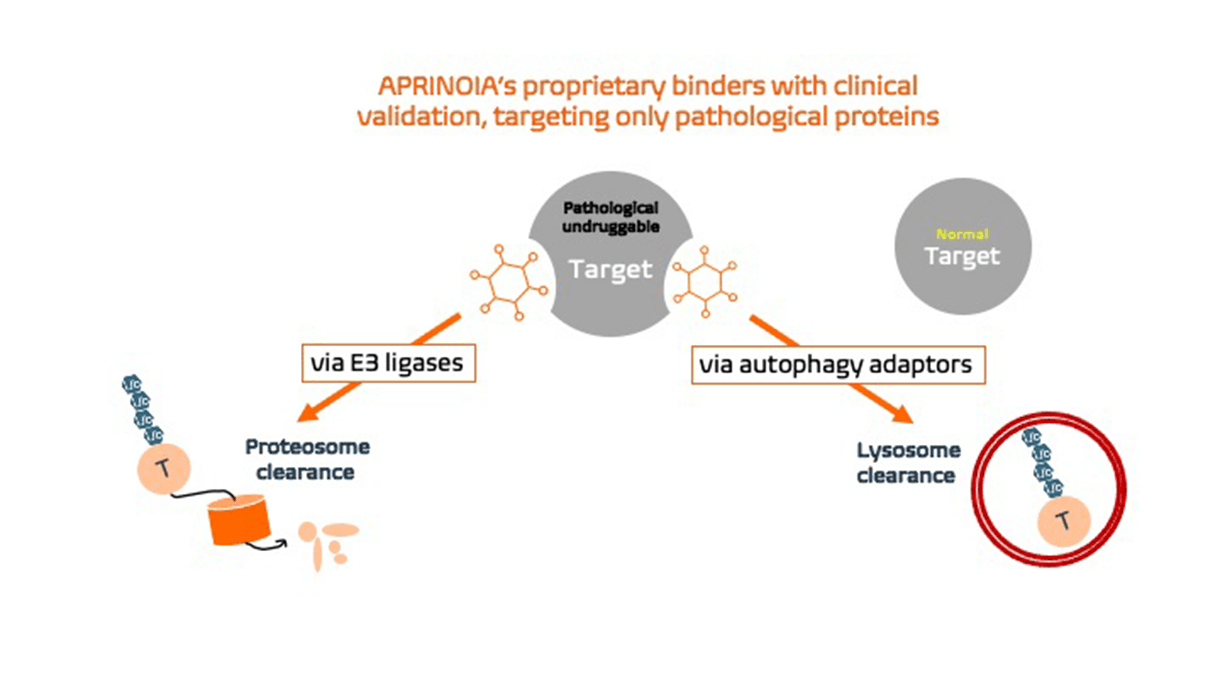
APRINOIA has generated protein degraders leveraging multiple binders and clearance pathways. Inspired by Proteolysis targeting chimerics (PROTAC) technology, our dual-functional degraders comprise an E3-ligase ligand and a target binding component, engaging pathological tau aggregates to the ubiquitin-proteasome system for further degradation. Our unique binders are designed based on recognizing the specific binding pockets only present in toxic tau aggregates.
This unique feature ensures that our degrader candidates only target disease forms of pathological protein aggregates, while leaving the native counterparts untouched to avoid possible side effects. We are investigating the utility of these disease-specific degraders for common tau-related disorders, such as AD, as well as rare primary tauopathies, such as PSP, FTD, and orphan diseases. Given the heterogeneous nature underlying these disorders, patient selection becomes critical and will be addressed using the company’s proprietary diagnostic tools.